Analyzing Trinitite: A (Radioactive) Piece of Nuclear History
On July 16, 1945, the United States became the first country to successfully detonate an atomic weapon, signalling the beginning of a new era in warfare and in politics. This detonation took place in the middle of the New Mexico desert, with the bomb placed carefully atop a 100 foot tower. The bomb, nicknamed “Gadget”, had a yield equivalent to 20,000 tons of TNT. Just 24 days later, a functionally similar bomb (using Plutonium, unlike the Uranium bomb at Hiroshima) was dropped on Nagasaki.
No one was completely sure what would happen when Gadget went off. For a while, there was worry that the chain reaction would be unstoppable and react with the entire atmosphere. Before the test, Enrico Fermi took bets from some of the physicists and high ranking military personnel on whether the bomb would destroy the whole state of New Mexico, or the entire planet. The math seemed to show fairly conclusively that the world wouldn’t be destroyed, but a lot of the guards who didn’t know that became anxious. Kenneth Bainbridge, director of the Trinity Project, was not amused with Fermi scaring all the guards.
When the bomb was detonated, it left a crater of radioactive glass in the desert that was 10 feet deep and 1100 feet wide. About 240 people on the project directly watching the blast reported the early morning dawn being lit up brighter than full daylight for one to two seconds and felt a wave of heat roll over them that was “as hot as an oven”, even at a distance of 10 miles away. The shock wave took 40 seconds to propagate to the observers and was felt up to 100 miles away. The enormous mushroom cloud was 7.5 miles high. It was at this point that Bainbridge remarked to Oppenheimer, “Now we are all sons of bitches.” Oppenheimer later spoke his famous line, “Now I am become Death, the destroyer of worlds”, a quote from the Bhagavad Gita.
This famous picture was taken 16 milliseconds after the Trinity bomb exploded. 
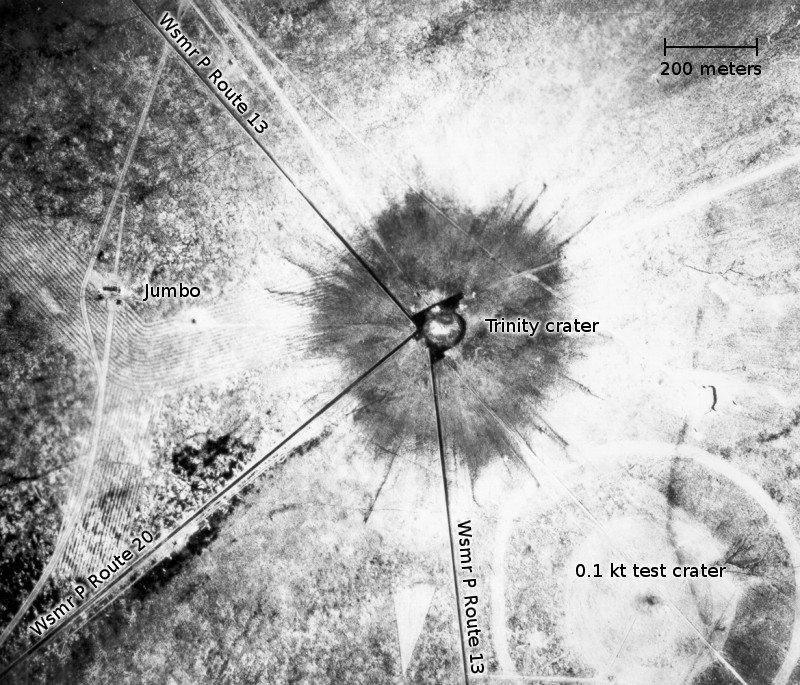
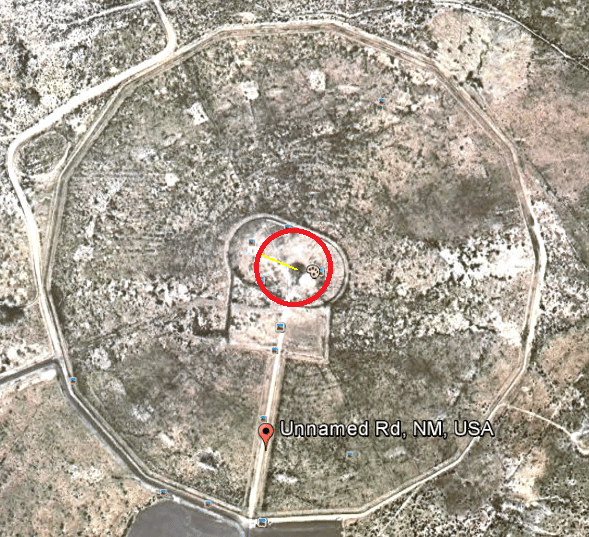
 That’s the hypocenter of the blast. Once the public found out about the bomb and where it was detonated (sometime during the late 40’s), they began traveling to the site and collecting the glass as souvenirs for themselves and to sell to tourists and collectors. This area was still lightly radioactive, and the government didn’t like the fact that people were carting off lots of the stuff or sniffing around their test sites. In 1953, the government bulldozed the site, burying any glass that was left and fenced off the area. A law was passed making it illegal to collect samples from the area. The only exception was that it was legal to buy and sell the glass that had already been collected and was already on the market. People began calling the collectible glass “Trinitite”.
That’s the hypocenter of the blast. Once the public found out about the bomb and where it was detonated (sometime during the late 40’s), they began traveling to the site and collecting the glass as souvenirs for themselves and to sell to tourists and collectors. This area was still lightly radioactive, and the government didn’t like the fact that people were carting off lots of the stuff or sniffing around their test sites. In 1953, the government bulldozed the site, burying any glass that was left and fenced off the area. A law was passed making it illegal to collect samples from the area. The only exception was that it was legal to buy and sell the glass that had already been collected and was already on the market. People began calling the collectible glass “Trinitite”.
The Artifact
You can still buy Trinitite today, on places like eBay or from mineral collectors. You can also buy it from United Nuclear, which is where I got my sample. United Nuclear makes two claims that I wanted to verify: that the sample was real and that the radiation level was safe. Apparently lots of people sell fake Trinitite, because it’s easy to fake and people will buy it. United Nuclear says that they check all the Trinitite they buy with a mass spectrometer to verify authenticity. Here’s what my sample looks like:  As an undergrad at Georgia Tech, I have access to some unique resources. One of my friends happens to be studying Nuclear and Radiological Engineering, so I asked him if he could measure my sample with a Geiger counter to make sure I wouldn’t get cancer or to confirm that I would get super powers with this thing sitting in my room. He grinned and said, “Oh, we can do better than that.”
As an undergrad at Georgia Tech, I have access to some unique resources. One of my friends happens to be studying Nuclear and Radiological Engineering, so I asked him if he could measure my sample with a Geiger counter to make sure I wouldn’t get cancer or to confirm that I would get super powers with this thing sitting in my room. He grinned and said, “Oh, we can do better than that.”
The Experiment
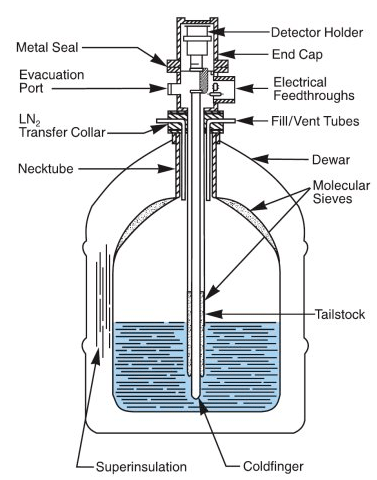
After talking to his professor, he got permission to run a bunch of tests on my sample for his lab class. It turns out that a Geiger counter wouldn’t be able to tell me very much about the sample, including how dangerous it really was. Instead, a high purity Germanium (HPGe) detector was used. HPGe detectors are large, expensive machines that must be cooled with liquid nitrogen. Here’s a cross section of a commercially available detector:
Because the detector is so cold, electrons have a lower probability of escaping, which allows for a higher resolution and higher efficiency. The reason Germanium is used is because of its useful properties as a semiconductor. As incoming radiation hits the Germanium, it creates free electrons and holes. An electric field across the Germanium causes all of the electrons to get pushed to one side, creating a current. This current is proportional to the number of holes created, which is proportional to the energy of the incoming radiation. This current is read with a Multiple Channel Analyzer (MCA) that bins the energy from the detector into multiple channels. The efficiency of an MCA changes based on the energy range, so they must be calibrated with known radiation sources before use. To determine if my sample was really from the Trinity test, its activity can be compared to the activity of specific radionuclides measured from glass collected at several other test sites. Gamma spectroscopy can be used to produce a plot that’s a little easier to visualize. To perform the experiment, the MCA was set at 4096 channels with a live time of 120 seconds and calibrated. The sample was placed in the shield surrounding the detector and measurements were taken for 80,000 seconds (22 hours). The sample was then removed and a background measurement was taken for 80,000 seconds.
Results
Here are the results, along with data from other test sites (as of 2013): 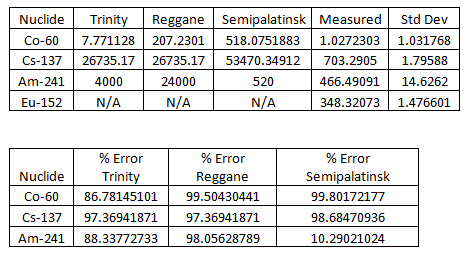 Note that percent error here is not standard deviation, it’s how much the measured sample deviates from a known sample. Here is the measured spectrum:
Note that percent error here is not standard deviation, it’s how much the measured sample deviates from a known sample. Here is the measured spectrum: 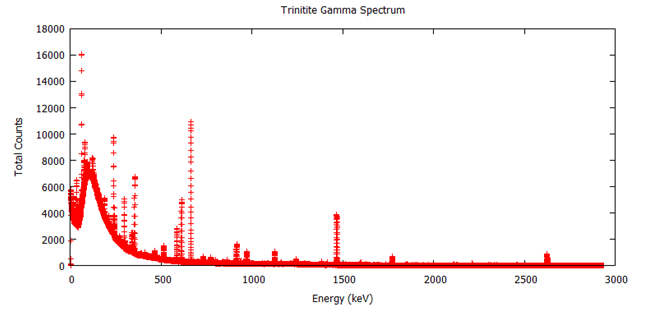 Analysis
Analysis
The interesting peaks here are Cs-137 at 661.5 keV, Am-241 at 59.4 keV, and K-40 at 1400 keV. The specific activity of Co-60 is very low, which is the biggest clue that the glass was collected from Trinity, rather than another location. For example, at Reggane, Co-60 is 207.2301 Bq/kg as of 2013, while Trinity is closer to 7.771128 Bq/kg. The activity we see here is 1.0 ± 1.0 Bq/kg, which suggests that my sample from United Nuclear is real and came from Trinity! The other thing this data lets us do is see how dangerous the radiation from my sample is. To do this, we simply sum up the counts collected at every energy and divide by the amount of time the sample was measured to get an energy indiscriminate counts per minute. It turns out my sample has a total gamma activity of 1183.29 CPM ± 5.43 CPM. So how do we know if that’s dangerous? That’s a little difficult to answer, because CPM is relative. The type and energy of the radiation matters enormously. This analysis only looked at gamma radiation, since that’s the kind of radiation that is extremely penetrating and difficult to shield. However, gamma radiation is not as damaging as other forms of radiation of the same energy, like neutron radiation. My sample also emits alpha and beta particles, but they are very short range and are easy to block. Alpha radiation can’t penetrate your clothes and beta radiation can be stopped with only a few millimeters of aluminum. So, ignoring alpha and beta radiation, the damage gamma radiation does increases with energy, and we have a wide range of energies in this sample. If we assume all the gamma rays are 661.5 keV from the Cs-137 and you are 1 cm from the source:
The attenuation of photons in water (which closely approximates human tissue) is: 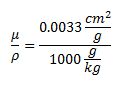 Multiplying those two numbers gives 5.49*10^-13 Gray/second, or 1.73*10^-5 Gray/year. The conversion factor from Grays to Sieverts is 1:1 for photons. 100 Rem is a Sievert. This means that if you keep this sample 1 cm away from you for the rest of your life you will receive 1.73 milliRem per year.
Multiplying those two numbers gives 5.49*10^-13 Gray/second, or 1.73*10^-5 Gray/year. The conversion factor from Grays to Sieverts is 1:1 for photons. 100 Rem is a Sievert. This means that if you keep this sample 1 cm away from you for the rest of your life you will receive 1.73 milliRem per year.
Bonus Points
There’s one more really neat piece of information we can pry out of the data. A naturally occurring isotope in soil is Eu-151. When Eu-151 captures a neutron from, say, a nuclear explosion, it turns into Eu-152. That’s a great way to estimate the neutron fluence (which is neutron flux integrated over time). So, if we can estimate the neutron fluence of my sample, and since we know how neutrons are emitted by the explosion at different distances, we can estimate how far away from ground zero my sample came from.
The math gets a little hairy here, so get ready.
Eu-151 undergoes an (n,γ) to make Eu-152 (it captures a neutron). The specific activity of Eu-152 is given by this equation:
Where φth is the thermal neutron fluence rate (n cm-2 s-1), a is the isotopic abundance of Eu-151, NA is Avogadro’s constant, M is the atomic mass of europium, c is the Eu concentration in the soil, σth is the thermal microscopic cross section of the (n,γ) reaction, CR is the cadmium ratio for a nuclear reactor, and T1/2 is the half-life of Eu-152. From [3], σth = 5300 barns, CR = 43, and c = 1.2 ppm. The cadmium ratio approximately accounts for the contribution of fast neutrons to the production of Eu-152.
After calculating the flux, one can solve for the distance from ground zero via this equation:
The specific activity of the Eu-152 was calculated by dividing the net counts by the duration of time over which it was counted, the mass of the sample, the efficiency for the energy of interest, and the branching ratio for the decay mode of interest.
After decay correcting the specific activity to the time of the explosion (68 years) and solving for the flux:
Solving for the distance from the center of the explosion:
Solving for the distance from ground zero:
So my sample was collected about 76 meters from ground zero. That means it came from somewhere along this circumference:
Future Work
Some kinds of Trinitite formed from sand on the ground turning to glass, and other kinds formed from sand being drawn up into the explosion, turning to glass, and then raining back down. If my sample was formed on the ground, we would expect the distribution of radionuclides would have an approximately continuous gradient. If it formed in the air, there would be a discontinuity in the distribution. This could be estimated with beta spectroscopy on each side of a sample. If the activity is spatially uniform, then the radionuclides are uniformly distributed. For this experiment, only gamma spectroscopy on one side was performed, so it’s unclear how my sample formed. However, a lot of the pieces in my sample appear large and flat, like they flaked off of the surface of the desert, so I would hypothesize that it formed on the ground. I would expect them to be rounder if they formed in the air.
Conclusion
It’s interesting to think of all the time and effort and money that went in to making the device that created Trinitite. It’s like I have a little vial of second order creations by Fermi and Feynman and Oppenheimer. I learned a lot about radiation physics and now I have a nice test radiation source for other projects that is pretty well characterized.
References
None of this would be possible without Nicholas Piper, who collected the data, performed analysis, and answered my questions. Also, thank you to his lab partners, Catherine Bartgis, Akshat Bhatnagar, and Benjamin Bane.
1. Tsoulfanidis, Nicholas, and Sheldon Landsberger. Measurement and Detection of Radiation. 3rd ed. Boca Raton, FL: Taylor and Francis Group, 2011. 384-385. Print.
2. Belloni F, Himbert J, Marzocchi O, Romanello V. “Investigating incorporation and distribution of radionuclides in trinitite.” May 5, 2011. Journal of Environmental Radioactivity, 2011.
3. Parekh P, Semkow T, Torres M, Haines D, Cooper J, Rosenberg P, Kitto M. “Radioactivity in Trinitite six decades later.” Wadsworth Center. University at Albany. Journal of Environmental Radioactivity, 2005.
Other interesting Trinity videos
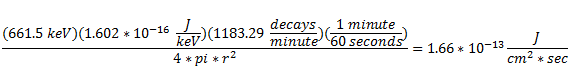

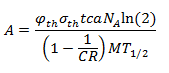
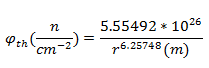
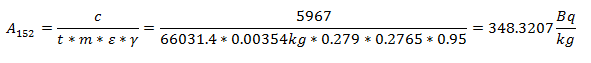
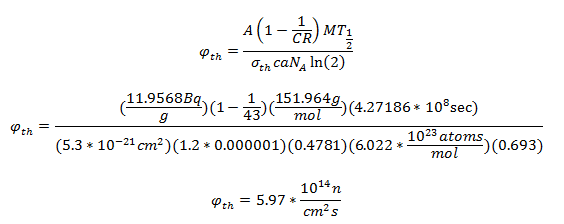
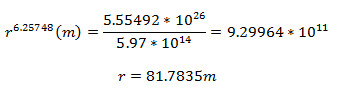
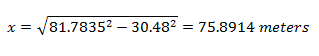
Pingback: “Grappling with the monsters of the American Psyche” (part 1) by Bradford Riley | Rileybrad's Blog
Pingback: Made By The Most Powerful Manmade Force Ever | Wow! Now I Know
Pingback: Two Fingers Media Trinitite: The radioactive rock buried in New Mexico before the Atari games
Pingback: BrokenPla.net - The Important Eclectic News Syndicate
Pingback: Trinitite: How the First Nuclear Bomb Turned Sand to Glass - Technology
Pingback: Trinitite | PhysicsOpenLab
Pingback: Trinitite | PhysicsOpenLab
Pingback: Today in Chemistry History – The Trinity Nuclear Bomb Test – Compound Interest